Current Research Projects at CSVRC
High Speed Angiography
Dr. Rudin was awarded an NIH RO1 Grant for "High Speed Angiography at 1000 frames per second." The grant was in the top 1 percentile and received an impact score of 19 in the first submission. The long-term goal of his project is to provide unprecedented 1000 frame per second angiography based on single- photon-counting detectors but with standard x-ray sources (a total capability presently unavailable to clinicians) to enable improved diagnostic and interventional patient care. The research team expects to rigorously test the High Speed Angiography system on patient-specific pathological phantoms to evaluate the potential impact on clinical decision making primarily in neuro-endo vascular procedures such as treatment of and predictions for ischemic stroke due to vasospasm following hemorrhagic stroke. The results of this project should lead toward future clinical testing of this new imaging concept with the capability to vastly improve vascular image-guided interventional procedures by providing clinicians, even while treating the patient, to visualize for the first time the intricate details of blood flow that can be so crucial to the determination of clinical procedure outcome. We expect that, just as our previous high-spatial-resolution-detector NIH- funded project has been translated to practice, this HSAngio project will eventually become the standard state-of-the art in diagnostic and interventional imaging as well.
Left/above video: simulated flow through the internal carotid artery with saccular aneurysm, shown at 1000fps
Patient Skin Dose Tracking
The Dose Tracking System (DTS) provides the interventional staff with real-time feedback of the patient’s skin dose distribution during fluoroscopically-guided procedures by providing a color-coded mapping of dose on a patient graphic. The DTS was developed in response to an FDA advisory on possible severe skin dose effects from long interventional procedures. The system is currently licensed to Canon Medical Systems with world-wide distribution and it received the Minnie Award as the Best New Radiology Software in 2014.

Scattered Radiation Display System
A Scattered Radiation Display System (SDS) is being developed to make the staff aware of the current radiation distribution in the procedure room so they can take appropriate action in avoiding high dose rate areas and in proper use of radiation shielding. The virtual display of room scatter is updated as the exposure techniques and beam geometry changes during the procedure. In addition, individual staff dose values are displayed and recorded using human recognition software and staff tracking.

Eye Lens Dose Tracking
There has been increasing concern for patient cataractogenesis due to radiation dose received during neuro-interventional procedures. We are developing a system to track the eye lens dose during the procedure so that appropriate action can be taken to avoid excessive exposure to the eyes. The cumulative lens dose values are presented on the DTS display to provide real-time feedback and a warning when thresholds for injury are being reached. As part of this system, the location of the head in the beam is tracked automatically through AI evaluation of the fluoroscopic images.

Radiation Safety Training Using Virtual Reality
A virtual world is created in Unity3D to reproduce the interventional procedure room with 3D stereolithographic (STL) meshes representing the patient, C-Arm gantry, and patient table. Using an Oculus Go headset, the changes in procedure room scattered radiation distribution can be visualized for either a post-procedure review of a prior patient procedure recorded as a CAN bus log file or as a virtual procedure in which the trainee can observe the effect on scatter of changes the trainee virtually makes in the conduct of a procedure
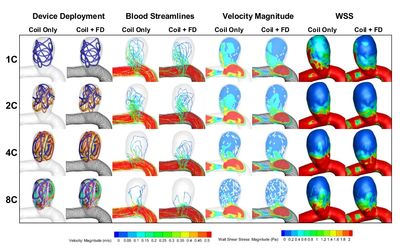
Computational Fluid Dynamics
The management of Intracranial aneurysms is challenging. Clinicians are routinely faced with the dilemma whether or not to treat the patient.
Using a growing database of patient-specific aneurysms (currently over 400 cases, we have built 3 statistical aneurysm rupture status stratification models based on morphology, hemodynamics and the combination of the two categories.
These rupture status prediction models are validated and demonstrated the prediction value for unruptured aneurysms.

Parametric Imaging
Parametric imaging, also referred to as colored coded DSA, is done by analyzing the contrast behavior at each pixel in the image. Since the early development of DSA there were many attempts to incorporate the temporal information of the contrast propagation. Such temporal information could be used to create a blood flow parametric description over the entire imaged vasculature. Despite these attempts this imaging processing method did not become clinical reality. The delays were due to various considerations such as computational speed, flow dependence on injection technique or limited knowledge of flow parameters with the patient physiology. The computational aspect meanwhile has been addressed using advanced hardware and advanced programming techniques. The value of individual pixels is tracked in each frame and a time density curve is built. Each curve is analyzed and curve parameters are calculated at each pixel. Various matrices containing parameters such as TTP, MTT, Arrival Time etc. can be calculated and later displayed using a colored mapping convention.

Vessel Wall Enhancement
Vessel wall enhancement (VWE) is a phenomenon observed in contrast-enhanced MRI images where the wall exhibits contrast enhancement in specific parts of the aneurysm wall after the injection of gadolinium based contrast. This has been correlated with inflammatory cell infiltration, neovascularization and presence of vasa-vasorum all of which are all characteristics of high-risk aneurysmal lesions. However, objective quantification of VWE is currently a challenge. We have developed an objective pipeline for VWE quantification and 3D visualization. Additionally, we also compute patient specific hemodynamic parameters and investigate underlying correlations to VWE to better understand the biology behind VWE. Our goal is to validate this on human longitudinal studies and potential animal models.

Image Based Biomarkers
Mechanical Thrombectomy (MT) has been the choice of treatment for clot retrieval for treatment of patients with Acute Ischemic Stroke. Overall first pass success rates of MT procedures are still ~30%, with choices on which MT device to use is mostly based on clinician’s experience. We seek to develop computer vision based Artificial Intelligence (AI) tools for automated evaluation of pre-MT images of patients to provide valuable information to the clinician to help them make the best patient-specific choice of therapy. Our research also extends to employing histopathological information from clots retrieved using MT to help explain the AI models and potentially correlate them to the actual biology of the clots.

In Silico Endovascular Interventions
Computer models and simulations are powerful tools to guide bioengineers and clinicians towards more effective treatment devices and techniques. Our in silico testbed of endovascular interventions is capable of generating and testing various treatment scenarios and device designs in a time- and cost-effective manner. Using our medical imaging database, we simulate real human vasculature complications, like occluding clots, and "test" endovascular treatment devices on them, like stent-retrievers. Our simulation results show high reliability and accuracy.
